1 对象与方法
1.1 研究对象
1.2 超声及超声造影检查
1.3 图像分析
1.4 联合CEUS的O-RADS分类
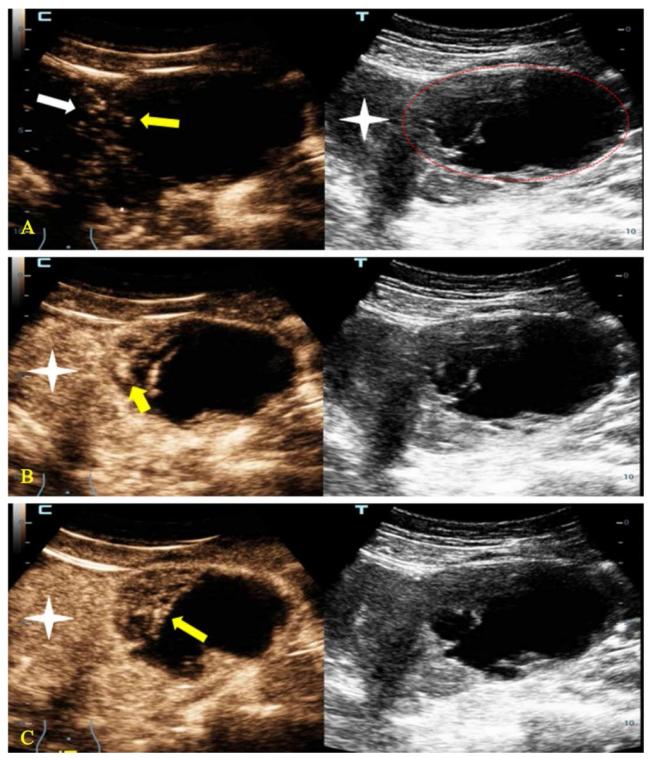
图1 一例35岁高级别卵巢囊腺癌患者的超声检查示例图注:A~C中,左方为超声造影图,右方为同一平面的超声图。A图显示子宫左侧一个O-RADS 4类的囊实性肿块(红色线条区),超声造影图显示肿块内造影剂到达时间(黄色箭头)与子宫肌层造影剂到达时间(白色箭头)基本相同。B图显示动脉期肿块内部增强最强区域(黄色箭头)要高于子宫肌层(星号)。C图显示消退期肿块(黄色箭头)与子宫肌层(星号)相比有消退。该肿块因超声造影积分为5分而升级其风险分层至O-RADS 5类。 Figure 1 Example of ultrasound examination in a 35-year-old patient with high-grade ovarian cystadenocarcinoma |
1.5 研究方法
1.6 统计学方法
2 结果
2.1 123例卵巢附件肿块的病理类型分布
表1 123例卵巢附件肿块的病理类型分布Table 1 Pathological distribution of 123 cases of ovarian adnexal masses |
| 病理类型(良性) | 肿块/n(%) | 病理类型(恶性) | 肿块/n(%) |
|---|---|---|---|
| 卵巢囊腺瘤 | 46(48.9) | 卵巢囊腺癌 | 13(44.8) |
| 成熟畸胎瘤 | 23(24.4) | 交界性囊腺瘤 | 10(34.5) |
| 内膜异位囊肿 | 15(16.0) | 子宫内膜样腺癌 | 2(6.9) |
| 卵巢纤维瘤 | 5(5.3) | 未成熟畸胎瘤 | 1(3.4) |
| 卵泡膜纤维瘤 | 2(2.1) | 卵巢癌肉瘤 | 1(3.4) |
| 卵巢囊肿 | 3(3.2) | 卵巢颗粒细胞瘤 | 1(3.4) |
| 卵巢无性细胞瘤 | 1(3.4) | ||
| 总计 | 94(100) | 总计 | 29(100) |
2.2 卵巢附件肿块恶性组与良性组的一般资料比较
表2 卵巢附件肿块恶性组与良性组的一般资料比较Table 2 General information comparison between malignant and benign groups of ovarian adnexal masses |
| 一般资料 | 恶性组(n=29) | 良性组(n=94) | χ ²/ Z值 | P值 |
|---|---|---|---|---|
| 年龄/岁 | 42(34,53) | 37(32,44) | 1.637 | 0.102 |
| 绝经/n(%) | 10(34.5) | 13(13.8) | 6.218 | 0.013 |
| 肿块最大径/cm | 8.0(6.0,10.9) | 6.0(4.0,10.1) | 2.141 | 0.032 |
| 腹水/n(%) | 15/29(51.7) | 7/94(7.4) | 29.584 | <0.001 |
| O-RADS分类/n(%) | 38.176 | <0.001 | ||
| 3 | 1(3.4) | 34(36.2) | ||
| 4 | 15(51.7) | 57(60.6) | ||
| 5 | 13(44.8) | 3(3.2) |
2.3 卵巢附件肿块恶性组与良性组的超声造影特征比较
表3 卵巢附件肿块恶性组与良性组的超声造影特征比较Table 3 Comparison of contrast-enhanced ultrasound characteristics between malignant and benign groups of ovarian adnexal masses |
| 超声造影特征 | 恶性组(n=29) | 良性组(n=94) | χ 2值 | P值 |
|---|---|---|---|---|
| 增强时间/n(%) | 47.738 | < 0.001 | ||
| 等或早于子宫肌层 | 25(86.2) | 16(17.0) | ||
| 晚于子宫肌层 | 4(13.8) | 78(83.0) | ||
| 增强强度/n(%) | 26.434 | < 0.001 | ||
| 等或高于子宫肌层 | 22(75.9) | 20(21.3) | ||
| 低于子宫肌层 | 7(24.1) | 51(54.3) | ||
| 无增强 | 0(0) | 23(24.5) | ||
| 造影增强是否快进/n(%) | 68.170 | < 0.001 | ||
| 是 | 21(72.4) | 4(4.3) | ||
| 否 | 8(27.6) | 90(95.7) | ||
| 造影增强是否快退/n(%) | 51.659 | < 0.001 | ||
| 是 | 22(75.9) | 9(9.6) | ||
| 否 | 7(24.1) | 85(90.4) |
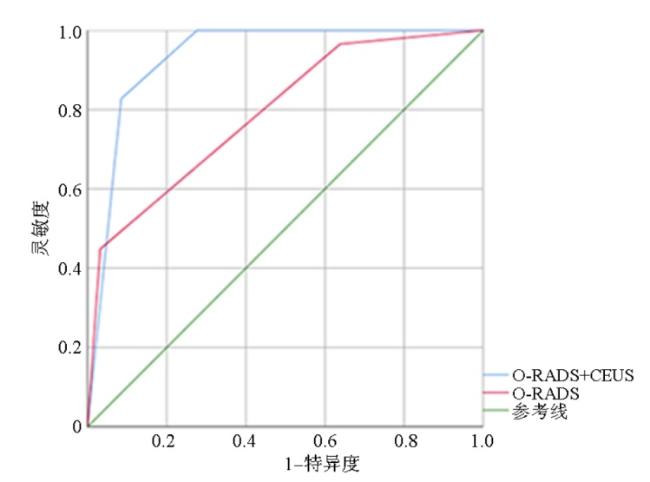
2.4 O-RADS+CEUS与O-RADS对恶性肿瘤的诊断效能比较
2.5 观察者间一致性分析
表4 观察者间一致性结果Table 4 Results of inter observer consistency |
| 观察者2结果 | 观察者1结果 | κ | |||
|---|---|---|---|---|---|
| O-RADS 3 | O-RADS 4 | O-RADS 5 | |||
| O-RADS分类/n(%) | O-RADS 3 | 32(26.0) | 4(3.3) | 0(0) | |
| O-RADS 4 | 2(1.6) | 67(54.5) | 3(2.4) | 0.840 | |
| O-RADS 5 | 1(0.8) | 1(0.8) | 13(10.6) | ||
| 早或等于子宫肌层 | 晚于子宫肌层 | ||||
| 增强时间/n(%) | 早或等于子宫肌层 | 38(30.9) | 7(5.7) | 0.821 | |
| 晚于子宫肌层 | 3(2.4) | 75(61.0) | |||
| 等或高于子宫肌层 | 低于子宫肌层 | 无增强 | |||
| 增强强度/n(%) | 等或高于子宫肌层 | 38(30.9) | 5(4.1) | 0(0) | |
| 低于子宫肌层 | 6(4.9) | 51(41.5) | 3(2.4) | 0.795 | |
| 无增强 | 0(0) | 2(1.6) | 20(16.3) | ||
| 是 | 否 | ||||
| 动脉期快速增强/n(%) | 是 | 20(16.3) | 9(7.3) | 0.668 | |
| 否 | 5(4.1) | 89(72.4) | |||
| 是 | 否 | ||||
| 是否快速消退/n(%) | 是 | 25(20.3) | 8(6.5) | 0.704 | |
| 否 | 6(4.9) | 84(68.3) | |||