1 对象与方法
1.1 研究对象
1.2 方法
1.2.1 基线临床资料
1.2.2 多导睡眠呼吸监测
1.2.3 冠状动脉造影
1.2.4 冠状动脉CT血管显像
1.3 统计学方法
2 结 果
2.1 训练集和验证集的OSA患者基线资料对比
表1 训练集与验证集的OSA患者基线资料对比Table 1 Comparison of baseline data of patients with OSA between training set and validation set |
| 变 量 | 总体(n=713) | 训练集(n=500) | 验证集(n=213) | t/ χ 2/Z值 | P值 |
|---|---|---|---|---|---|
| 男性/n(%) | 610(85.55) | 435(87.00) | 175(82.16) | 2.832 | 0.092 |
| 年龄/岁 | 48.84±9.82 | 48.96±9.94 | 48.55±9.58 | 1.551 | 0.121 |
| BMI/(kg/m2) | 27.44(24.62,30.25) | 27.39(24.56,30.34) | 27.68(25.15,30.15) | -1.341 | 0.237 |
| 高血压/n(%) | 297(41.65) | 203(40.60) | 94(44.13) | 0.766 | 0.381 |
| 糖尿病/n(%) | 269(37.73) | 199(39.80) | 70(32.86) | 3.059 | 0.080 |
| 吸烟史/n(%) | 364(51.05) | 259(51.80) | 105(49.30) | 0.375 | 0.540 |
| 饮酒史/n(%) | 336(47.12) | 240(48.00) | 96(45.07) | 0.514 | 0.473 |
| ALT/(U/L) | 26.51(17.89,41.60) | 26.29(17.67,42.13) | 26.75(18.81,40.52) | -0.633 | 0.527 |
| AST/(U/L) | 21.30(17.75,28.40) | 21.35(17.30,29.00) | 21.30(17.95,27.90) | -0.068 | 0.946 |
| 血清肌酐/(μmol/L) | 75.38±16.50 | 74.81±16.51 | 76.58±15.66 | -1.326 | 0.185 |
| 血尿酸/(μmol/L) | 376.43±90.31 | 377.43±93.28 | 376.54±83.90 | 0.120 | 0.904 |
| 总胆固醇/(mmol/L) | 4.59(4.04,5.23) | 4.56(4.04,5.18) | 4.65(4.02,5.31) | -0.588 | 0.557 |
| 甘油三酯/(mmol/L) | 1.90(1.33,2.81) | 1.92(1.35,2.84) | 1.81(1.25,2.73) | -1.025 | 0.305 |
| HDL-C/(mmol/L) | 1.07(0.89,1.30) | 1.05(0.89,1.28) | 1.11(0.89,1.32) | -1.298 | 0.194 |
| LDL-C/(mmol/L) | 2.54(1.98,3.13) | 2.53(1.98,3.09) | 2.55(1.99,3.22) | -0.275 | 0.784 |
| FBG/(mmol/L) | 5.20(4.62,5.80) | 5.21(4.60,5.80) | 5.14(4.73,5.79) | -0.144 | 0.885 |
| 24 h收缩压/mmHg | 132(123,142) | 132(123,143) | 133(122,141) | -0.096 | 0.924 |
| 24 h舒张压/mmHg | 86(78,93) | 86(78,93) | 87(78,93) | -0.415 | 0.678 |
| MSaO2/% | 92.40(91.40,93.30) | 92.30(91.30,93.40) | 92.40(91.50,93.30) | -0.514 | 0.623 |
| LSaO2/% | 81.00(76.00,85.00) | 81.00(76.00,85.00) | 82.00(77.00,85.00) | -0.847 | 0.379 |
| AHI/(次/小时) | 18.90(9.20,25.00) | 18.80(9.10,25.30) | 18.90(9.30,24.60) | -0.162 | 0.835 |
| MLR | 0.23(0.19,0.28) | 0.23(0.18,0.28) | 0.23(0.18,0.29) | -0.500 | 0.617 |
| SIRI | 0.84(0.60,1.15) | 0.82(0.59,1.12) | 0.88(0.62,1.19) | -1.230 | 0.219 |
注:1 mmHg=0.133 kPa。 |
2.2 训练集中非冠心病患者和冠心病患者的基线资料对比
表2 训练集中是否合并冠心病的OSA患者组间基线资料对比Table 2 Comparison of the baseline data between the OSA patients with or without coronary heart disease in the training set |
| 变 量 | 非冠心病患者(n=355) | 冠心病患者(n=145) | t/ χ 2/Z值 | P值 |
|---|---|---|---|---|
| 男性/n(%) | 307(86.84) | 128(88.28) | 0.294 | 0.588 |
| 年龄/岁 | 47.56±9.22 | 52.02±10.8 | -4.220 | <0.001 |
| BMI/(kg/m2) | 27.20(24.46,29.75) | 27.72(24.98,30.87) | -2.128 | 0.035 |
| 高血压/n(%) | 124(34.93) | 79(54.88) | 16.321 | <0.001 |
| 糖尿病/n(%) | 127(35.77) | 72(49.66) | 8.279 | 0.004 |
| 吸烟史/n(%) | 182(51.52) | 77(51.30) | 0.139 | 0.709 |
| 饮酒史/n(%) | 174(49.01) | 66(45.52) | 0.504 | 0.478 |
| ALT/(U/L) | 26.60(18.00,41.68) | 26.00(17.08,42.75) | -0.737 | 0.461 |
| AST/(U/L) | 21.22(17.30,28.61) | 22.05(17.10,30.46) | -0.013 | 0.989 |
| 血清肌酐/(μmol/L) | 74.67±16.75 | 75.17±15.96 | -0.308 | 0.758 |
| 血清尿酸/(μmol/L) | 376.19±94.35 | 380.46±90.86 | -0.465 | 0.642 |
| 总胆固醇/(mmol/L) | 4.54(4.02,5.12) | 4.68(4.14,5.28) | -1.925 | 0.054 |
| 甘油三酯/(mmol/L) | 1.93(1.41,2.83) | 1.92(1.32,2.86) | -0.479 | 0.632 |
| HDL-C/(mmol/L) | 1.04(0.88,1.28) | 1.07(0.90,1.28) | -0.409 | 0.683 |
| LDL-C/(mmol/L) | 2.48(1.93,3.04) | 2.63(2.13,3.32) | -2.456 | 0.014 |
| FBG/(mmol/L) | 5.20(4.56,5.75) | 5.28(4.69,6.09) | -1.746 | 0.081 |
| 24 h收缩压/mmHg | 132(124,141) | 132(120,146) | -0.056 | 0.956 |
| 24 h舒张压/mmHg | 86(78,93) | 86(77,94) | -0.672 | 0.502 |
| MSaO2/% | 92.40(91.50,93.50) | 92.30(91.10,93.00) | -1.835 | 0.068 |
| LSaO2/% | 82.00(77.00,85.00) | 80.00(73.00,85.00) | -2.156 | 0.031 |
| AHI/(次/小时) | 18.60(8.50,23.20) | 21.57(10.48,28.63) | -3.753 | <0.001 |
| MLR | 0.22(0.18,0.27) | 0.25(0.19,0.32) | -3.561 | <0.001 |
| SIRI | 0.79(0.55,1.03) | 1.00(0.64,1.36) | -4.308 | <0.001 |
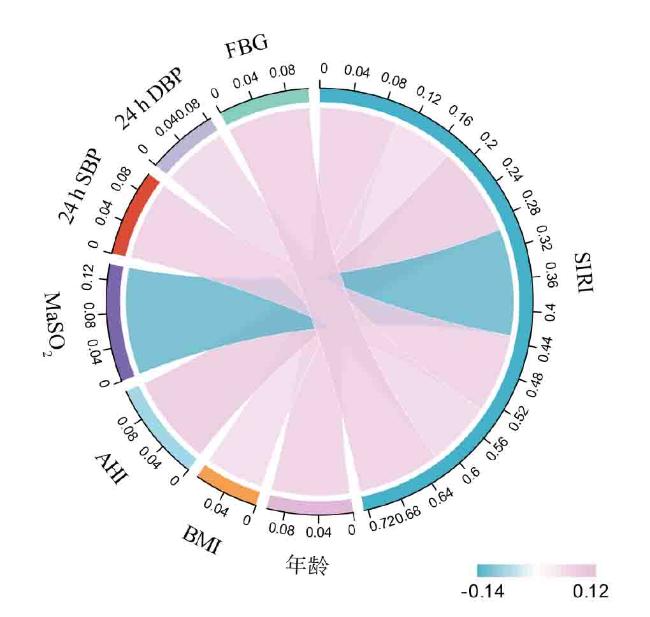
2.3 SIRI与OSA合并冠心病的各种相关因素的相关性分析
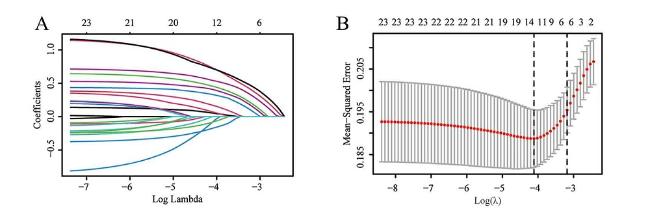
2.4 LASSO回归筛选OSA合并冠心病的预测因子
2.5 OSA患者合并冠心病风险影响因素的多因素Logistic回归分析
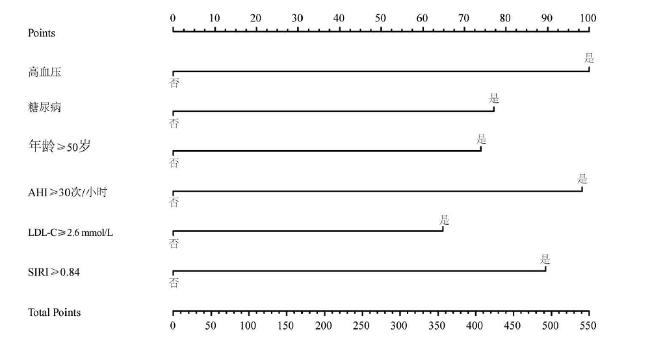
合并冠心病的危险因素(均P < 0.05)。见表3。
表3 OSA患者合并冠心病风险影响因素的多因素Logistic回归分析Table 3 Multivariate Logistic regression analysis of risk factors for OSA combined with CHD |
| 变 量 | β | SE | χ 2值 | P值 | OR值 | OR的95%CI | |
|---|---|---|---|---|---|---|---|
| 下限 | 上限 | ||||||
| 年龄≥50岁 | 0.665 | 0.215 | 9.589 | 0.002 | 1.947 | 1.277 | 2.969 |
| 高血压 | 0.901 | 0.216 | 17.381 | <0.001 | 2.462 | 1.612 | 3.761 |
| 糖尿病 | 0.695 | 0.207 | 10.382 | 0.001 | 2.003 | 1.313 | 3.057 |
| LDL-C ≥ 2.6 mmol/L | 0.584 | 0.215 | 7.351 | 0.007 | 1.793 | 1.176 | 2.735 |
| AHI ≥ 30次/小时 | 0.886 | 0.245 | 13.071 | <0.001 | 2.425 | 1.500 | 3.920 |
| SIRI ≥ 0.84×109/L | 0.806 | 0.217 | 13.788 | <0.001 | 2.240 | 1.463 | 3.428 |
2.6 OSA患者合并冠心病风险的列线图
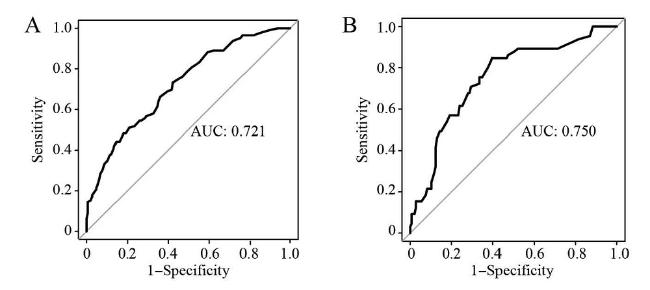
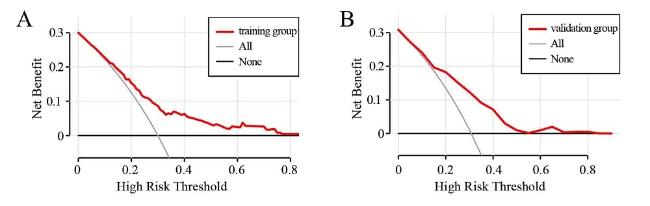
2.7 临床预测模型的验证和评价
图4 训练集与验证集的ROC曲线注:A为训练集,B为验证集。 Figure 4 The ROC curves for the training set and the validation set |
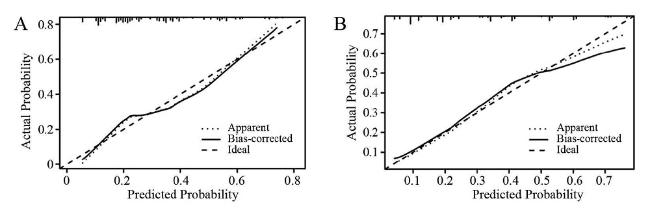
图5 训练集与验证集的校准曲线注:A为训练集,B为验证集。 Figure 5 Calibration curves for the training set and the validation set |
2.8 SIRI与OSAHS合并冠心病的传统危险因素间预测价值比较
表4 SIRI与OSAHS合并冠心病的传统危险因素间的预测价值比较Table 4 Comparison of predictive value between traditional risk factors and SIRI of OSAHS with CHD |
| 危险因素 | 灵敏度/% | 特异度/% | AUC | AUC的95%CI | |
|---|---|---|---|---|---|
| 下限 | 上限 | ||||
| 年龄+高血压+糖尿病+LDL-C+AHI | 63.4 | 56.1 | 0.677 | 0.626 | 0.727 |
| 年龄+高血压+糖尿病+LDL-C+AHI+SIRI | 69.7 | 61.7 | 0.719 | 0.671 | 0.766 |