1 材料与方法
1.1 材料
1.2 主要试剂与仪器
1.3 细胞培养与转染
1.4 实时荧光定量PCR法检测GCNT3的mRNA表达水平
1.5 细胞计数试剂盒-8法检测细胞增殖
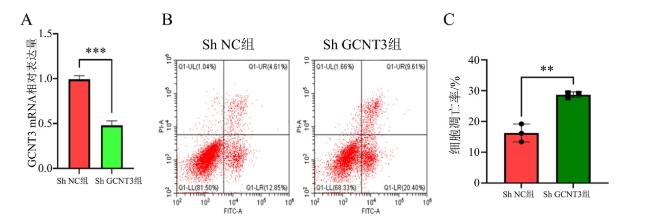
1.6 流式细胞仪检测H1650细胞凋亡
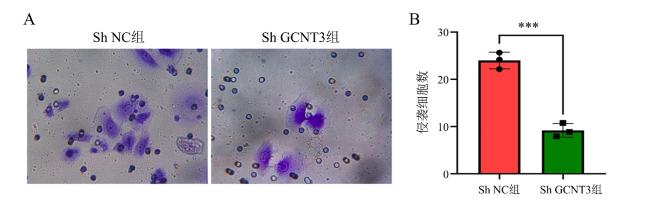
1.7 Transwell法检测H1650细胞的侵袭能力
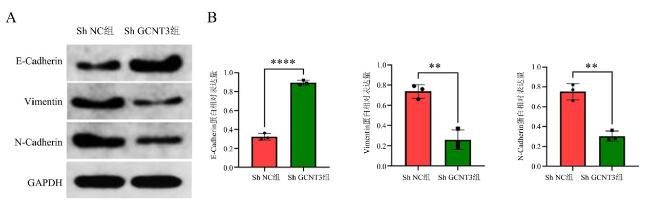
1.8 蛋白免疫印迹法检测上皮-间质转化相关蛋白的表达水平
1.9 统计学方法
2 结果
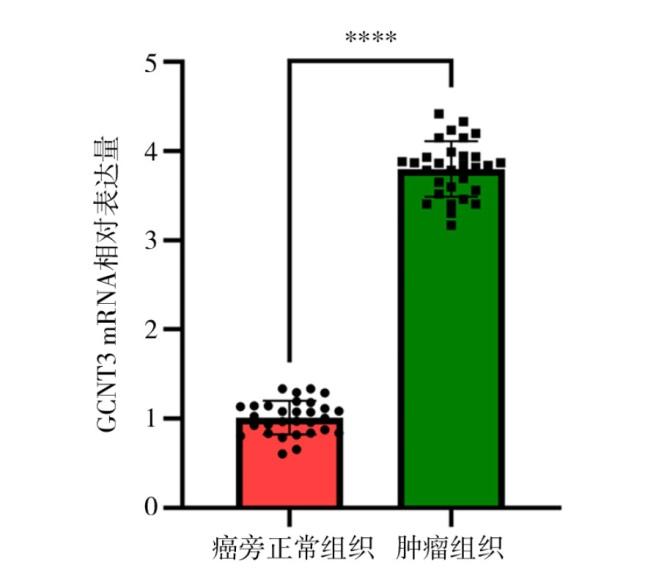
2.1 GCNT3在癌旁正常组织和肺腺癌组织中的表达情况
2.2 下调GCNT3对肺腺癌H1650细胞增殖水平的影响
表1 下调GCNT3对肺腺癌H1650细胞增殖水平的影响Table 1 The effect of downregulating GCNT3 on the proliferation level of lung adenocarcinoma H1650 cells |
| 组 别 | n | 细胞增殖率/% | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| Sh NC组 | 3 | 57.64±3.96 | 76.26±4.58 | 92.24±5.12 |
| Sh GCNT3组 | 3 | 42.32±3.72 | 62.21±4.62 | 73.31±4.86 |
| t值 | -4.88 | -3.74 | -4.64 | |
| P值 | 0.008 | 0.020 | 0.010 | |