1 对象与方法
1.1 研究对象
1.2 超声引导下甲状腺结节细针穿刺及活组织检查
2 结果
2.1 一般资料
表1 甲状腺结节患者的临床资料 单位:nTable 1 Clinical data of patients with thyroid nodules |
| 项 目 | 甲状腺嗜酸细胞 肿瘤(n=22) | 桥本甲状腺炎(n=17) | 甲状腺髓样癌(n=10) | 嗜酸细胞亚型甲状腺 乳头状癌(n=6) |
|---|---|---|---|---|
| 性别 | ||||
| 男 | 9 | 3 | 3 | 3 |
| 女 | 13 | 14 | 7 | 3 |
| 年龄 | ||||
| <40岁 | 9 | 7 | 4 | 2 |
| ≥40岁 | 13 | 10 | 6 | 4 |
| 结节位置 | ||||
| 左侧叶 | 9 | 7 | 5 | 5 |
| 右侧叶 | 13 | 10 | 6 | 0 |
| 峡部 | 0 | 0 | 0 | 1 |
| 结节大小 | ||||
| ≤5 mm | 0 | 4 | 1 | 0 |
| > 5~10 mm | 1 | 6 | 4 | 5 |
| > 10~20 mm | 9 | 2 | 2 | 1 |
| > 20 mm | 12 | 3 | 4 | 0 |
| 不详 | 0 | 2 | 0 | 0 |
| ACR-TIRADS评分 | ||||
| TR2级/3级(2~3分) | 1/8 | 2/3 | 0/1 | 0/0 |
| TR4级/5级(≥4分) | 8/5 | 1/9 | 6/4 | 3/3 |
| 不详 | 0 | 2 | 0 | 0 |
| TPO-Ab水平升高 | 15 | |||
| TG-Ab水平升高 | 17 | |||
| 血清降钙素 | ||||
| 正常 | 1 | |||
| 升高 | 6 |
注:甲状腺髓样癌为10例患者11份结节标本,7例术前检测了血清降钙素。 |
2.2 病理形态学特征
2.2.1 嗜酸细胞肿瘤的细胞病理形态学特征
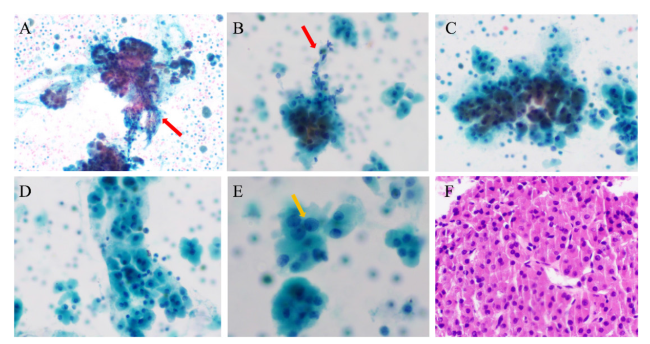
图1 嗜酸细胞肿瘤的细针穿刺细胞学检查结果注:A、B可见大片或小片状分布的嗜酸细胞及毛细血管穿越现象,红色箭头所指可见拖尾的血管内皮细胞;C、D可见散在或小片状分布的嗜酸细胞聚集现象;E可见嗜酸细胞胞浆丰富、浓厚,3~10个细胞呈群分布,黄色箭头所指可见核膜轻微不规则及小核仁;F可见嗜酸细胞平铺呈团排列,细胞间似以镶嵌状或推挤式排列。A~E为液基细胞学巴氏染色,F为细胞蜡块HE染色,A×100,B~D×200,E×400,F×200。 Figure 1 Fine-needle aspiration cytology of oncocytic thyroid neoplasm |
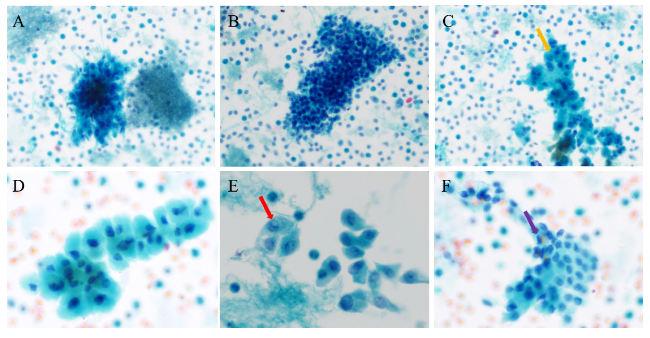
2.2.2 桥本甲状腺炎
2.2.3 甲状腺髓样癌
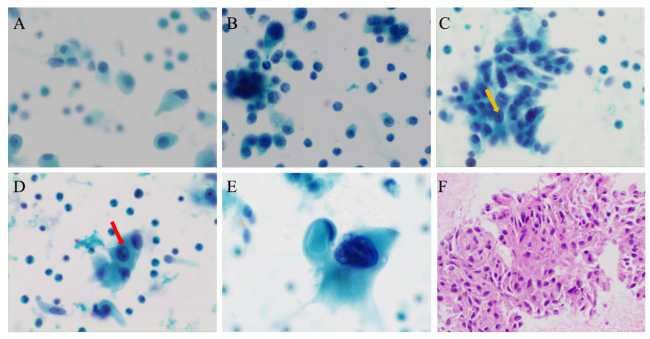
图3 甲状腺髓样癌的细针穿刺细胞学检查结果注:A可见以单个、散在分布的肿瘤细胞为主;B可见细胞聚集成小簇状,部分粉尘状细胞的核明显;C可见细胞形成小巢状,大小不一,形态多样,呈梭形、上皮样或圆形,黄色箭头所指可见小核仁;D红色箭头所指可见核内包涵体;E可见单个体积巨大的肿瘤细胞,胞浆丰富,嗜酸,核常常偏位,核染色质深;F可见细胞核扭曲、核形不规则,胞浆丰富、嗜酸,似有极向。A~E为液基细胞学巴氏染色,×400;F为细胞蜡块HE染色,×200。 Figure 3 Fine-needle aspiration cytology of medullary thyroid carcinoma |