 PDF(2676 KB)
PDF(2676 KB)


新冠肺炎患者和细菌性肺炎患者血清差异表达的细胞因子特征分析
罗斯威, 姚亚超, 钟丽梅, 胡亮杉, 肖威, 方晓琳, 曹东林
新医学 ›› 2022, Vol. 53 ›› Issue (5) : 372-378.
 PDF(2676 KB)
PDF(2676 KB)
 PDF(2676 KB)
PDF(2676 KB)
新冠肺炎患者和细菌性肺炎患者血清差异表达的细胞因子特征分析
Longitudinal characteristics of cytokine profiles in the peripheral blood of COVID-19 and bacterial pneumonia patients
目的 了解新型冠状病毒肺炎(新冠肺炎)患者与细菌性肺炎患者细胞因子的差异,探讨新冠肺炎免疫病理机制,为新冠肺炎提供新的治疗靶点。方法 收集轻型新冠肺炎患者(新冠肺炎组)、细菌性肺炎患者(细菌性肺炎组)和健康志愿者(健康对照组)3组各15份血清标本,进行48种细胞因子的检测,比较3组间细胞因子表达谱的差异。检测10例新冠肺炎患者在治疗不同时间点的血清标本中48种细胞因子表达,分析其与新冠肺炎患者的临床特征及疾病进展的相关性。结果 新冠肺炎组与细菌性肺炎组比较中,巨噬细胞迁移抑制因子(MIF)、生长调节致癌基因α(GROα)、粒细胞-巨噬细胞集落刺激因子(GM-CSF)和TNF-β在新冠肺炎组中上调(P均< 0.05)。而 IL-10、IL-18、IL-2受体α和IFN-γ诱导的单核细胞因子(MIG)在细菌性肺炎组中上调(P均< 0.05)。巨噬细胞集落刺激因子(M-CSF)在新冠肺炎患者住院治疗的第2周有明显的下调;单核趋化蛋白-1(MCP-1)的变化与新冠肺炎患者的核酸检测结果变化一致。结论 新冠肺炎患者和细菌性肺炎患者体内存在不同的细胞因子表达谱。M-CSF和MCP-1在新冠肺炎疾病进展中起着重要作用。
Objective To investigate the differences of cytokine profiles between patients with novel COVID-19 and bacterial pneumonia, unravel the immunopathological mechanism of COVID-19, and provide evidence for new therapeutic targets in COVID-19. Methods In this study, 15 serum samples were collected from mild COVID-19 patients (COVID-19 group), bacterial pneumonia patients (bacterial pneumonia group), and healthy controls (healthy control group), respectively. The expression profile of 48 cytokines was determined to clarify the differences among three groups. The expression levels of 48 cytokines at different time points were measured in serum samples from 10 patients with COVID-19 to analyze their correlation with clinical features and disease progression. Results In the comparation between the COVID-19 group and the bacterial pneumonia group, the expression levels of MIF, GROα, GM-CSF and TNF-β were upregulated in COVID-19 patients(all P < 0.05), whereas those of IL-10, IL-18, IL-2Rα and MIG were upregulated in patients with bacterial pneumonia(all P < 0.05). The expression level of M-CSF was significantly downregulated in COVID-19 patients at the second week of hospitalization. The changes in the expression level of MCP-1 were consistent with the nucleic acid test results of COVID-19 patients. Conclusions The cytokine profiles differ between patients with COVID-19 and bacterial pneumonia. In addition, M-CSF and MCP-1 play critical roles in the progression of COVID-19.
新型冠状病毒肺炎 / 细菌性肺炎 / 细胞因子 / 表达谱 {{custom_keyword}} /
COVID-19 / Bacterial pneumonia / Cytokine / Profile {{custom_keyword}} /
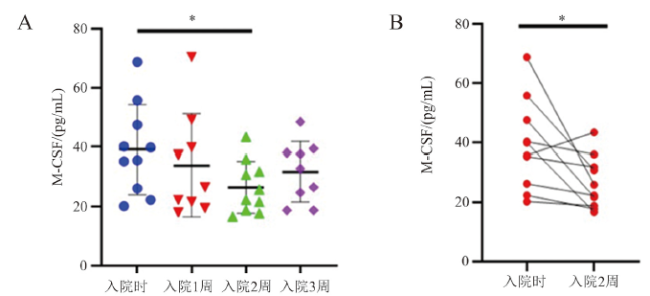
图4 M-CSF表达水平与新冠肺炎的疾病进展的相关性注:A为入院后不同时期新冠肺炎患者M-CSF的变化;B为入院时和入院2周的同一患者M-CSF水平配对分析;组间比较,*P < 0.05;n = 10。 |
| [1] |
An ongoing outbreak of pneumonia caused by a novel coronavirus, currently designated as the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was reported recently. However, as SARS-CoV-2 is an emerging virus, we know little about it. In this review, we summarize the key events occurred during the early stage of SARS-CoV-2 outbreak, the basic characteristics of the pathogen, the signs and symptoms of the infected patients as well as the possible transmission pathways of the virus. Furthermore, we also review the current knowledge on the origin and evolution of the SARS-CoV-2. We highlight bats as the potential natural reservoir and pangolins as the possible intermediate host of the virus, but their roles are waiting for further investigation. Finally, the advances in the development of chemotherapeutic options are also briefly summarized.© The author(s).
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [2] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [3] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [4] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [5] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [6] |
严丽, 李永胜. 新型冠状病毒肺炎重症患者的识别和处理策略. 新医学, 2020, 51(3): 161-167.
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [7] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [8] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [9] |
Coronavirus disease 2019 (COVID-19) is characterized by distinct patterns of disease progression that suggest diverse host immune responses. We performed an integrated immune analysis on a cohort of 50 COVID-19 patients with various disease severity. A distinct phenotype was observed in severe and critical patients, consisting of a highly impaired interferon (IFN) type I response (characterized by no IFN-β and low IFN-α production and activity), which was associated with a persistent blood viral load and an exacerbated inflammatory response. Inflammation was partially driven by the transcriptional factor nuclear factor-κB and characterized by increased tumor necrosis factor-α and interleukin-6 production and signaling. These data suggest that type I IFN deficiency in the blood could be a hallmark of severe COVID-19 and provide a rationale for combined therapeutic approaches.Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [10] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [11] |
The outbreak of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 was first reported in Wuhan, December 2019, and continuously poses a serious threat to public health, highlighting the urgent need of identifying biomarkers for disease severity and progression.We sought to identify biomarkers for disease severity and progression of COVID-19.Forty-eight cytokines in the plasma samples from 50 COVID-19 cases including 11 critically ill, 25 severe, and 14 moderate patients were measured and analyzed in combination with clinical data.Levels of 14 cytokines were found to be significantly elevated in COVID-19 cases and showed different expression profiles in patients with different disease severity. Moreover, expression levels of IFN-γ-induced protein 10, monocyte chemotactic protein-3, hepatocyte growth factor, monokine-induced gamma IFN, and macrophage inflammatory protein 1 alpha, which were shown to be highly associated with disease severity during disease progression, were remarkably higher in critically ill patients, followed by severe and then the moderate patients. Serial detection of the 5 cytokines in 16 cases showed that continuously high levels were associated with deteriorated progression of disease and fatal outcome. Furthermore, IFN-γ-induced protein 10 and monocyte chemotactic protein-3 were excellent predictors for the progression of COVID-19, and the combination of the 2 cytokines showed the biggest area under the curve of the receiver-operating characteristics calculations with a value of 0.99.In this study, we report biomarkers that are highly associated with disease severity and progression of COVID-19. These findings add to our understanding of the immunopathologic mechanisms of severe acute respiratory syndrome coronavirus 2 infection, and provide potential therapeutic targets and strategies.Copyright © 2020 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [12] |
Since December, 2019, Wuhan, China, has experienced an outbreak of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Epidemiological and clinical characteristics of patients with COVID-19 have been reported but risk factors for mortality and a detailed clinical course of illness, including viral shedding, have not been well described.In this retrospective, multicentre cohort study, we included all adult inpatients (≥18 years old) with laboratory-confirmed COVID-19 from Jinyintan Hospital and Wuhan Pulmonary Hospital (Wuhan, China) who had been discharged or had died by Jan 31, 2020. Demographic, clinical, treatment, and laboratory data, including serial samples for viral RNA detection, were extracted from electronic medical records and compared between survivors and non-survivors. We used univariable and multivariable logistic regression methods to explore the risk factors associated with in-hospital death.191 patients (135 from Jinyintan Hospital and 56 from Wuhan Pulmonary Hospital) were included in this study, of whom 137 were discharged and 54 died in hospital. 91 (48%) patients had a comorbidity, with hypertension being the most common (58 [30%] patients), followed by diabetes (36 [19%] patients) and coronary heart disease (15 [8%] patients). Multivariable regression showed increasing odds of in-hospital death associated with older age (odds ratio 1·10, 95% CI 1·03-1·17, per year increase; p=0·0043), higher Sequential Organ Failure Assessment (SOFA) score (5·65, 2·61-12·23; p<0·0001), and d-dimer greater than 1 μg/mL (18·42, 2·64-128·55; p=0·0033) on admission. Median duration of viral shedding was 20·0 days (IQR 17·0-24·0) in survivors, but SARS-CoV-2 was detectable until death in non-survivors. The longest observed duration of viral shedding in survivors was 37 days.The potential risk factors of older age, high SOFA score, and d-dimer greater than 1 μg/mL could help clinicians to identify patients with poor prognosis at an early stage. Prolonged viral shedding provides the rationale for a strategy of isolation of infected patients and optimal antiviral interventions in the future.Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences; National Science Grant for Distinguished Young Scholars; National Key Research and Development Program of China; The Beijing Science and Technology Project; and Major Projects of National Science and Technology on New Drug Creation and Development.Copyright © 2020 Elsevier Ltd. All rights reserved.
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [13] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [14] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [15] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [16] |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| [17] |
COVID-19, caused by SARS-CoV-2, has recently affected over 1,200,000 people and killed more than 60,000. The key immune cell subsets change and their states during the course of COVID-19 remain unclear. We sought to comprehensively characterize the transcriptional changes in peripheral blood mononuclear cells during the recovery stage of COVID-19 by single-cell RNA sequencing technique. It was found that T cells decreased remarkably, whereas monocytes increased in patients in the early recovery stage (ERS) of COVID-19. There was an increased ratio of classical CD14 monocytes with high inflammatory gene expression as well as a greater abundance of CD14IL1β monocytes in the ERS. CD4 T cells and CD8 T cells decreased significantly and expressed high levels of inflammatory genes in the ERS. Among the B cells, the plasma cells increased remarkably, whereas the naïve B cells decreased. Several novel B cell-receptor (BCR) changes were identified, such as IGHV3-23 and IGHV3-7, and isotypes (IGHV3-15, IGHV3-30, and IGKV3-11) previously used for virus vaccine development were confirmed. The strongest pairing frequencies, IGHV3-23-IGHJ4, indicated a monoclonal state associated with SARS-CoV-2 specificity, which had not been reported yet. Furthermore, integrated analysis predicted that IL-1β and M-CSF may be novel candidate target genes for inflammatory storm and that TNFSF13, IL-18, IL-2, and IL-4 may be beneficial for the recovery of COVID-19 patients. Our study provides the first evidence of an inflammatory immune signature in the ERS, suggesting COVID-19 patients are still vulnerable after hospital discharge. Identification of novel BCR signaling may lead to the development of vaccines and antibodies for the treatment of COVID-19.© The Author(s) 2020.
{{custom_citation.content}}
{{custom_citation.annotation}}
|
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
 PDF(2676 KB)
PDF(2676 KB)
 图1 仅在细菌性肺炎组中增多的细胞因子
图1 仅在细菌性肺炎组中增多的细胞因子 图2 同时在细菌性肺炎组和新冠肺炎组中增多的细胞因子
图2 同时在细菌性肺炎组和新冠肺炎组中增多的细胞因子 图3 仅在新冠肺炎组中水平发生变化的细胞因子
图3 仅在新冠肺炎组中水平发生变化的细胞因子 图4 M-CSF表达水平与新冠肺炎的疾病进展的相关性
图4 M-CSF表达水平与新冠肺炎的疾病进展的相关性 图5 3. SARS-CoV-2核酸由阳性转阴性的新冠肺炎患者 MCP-1和 M-CSF的动态变化
图5 3. SARS-CoV-2核酸由阳性转阴性的新冠肺炎患者 MCP-1和 M-CSF的动态变化/
| 〈 |
|
〉 |