急性大动脉闭塞性脑梗死是一种常见的、具有较高致残率和病死率的脑血管疾病,现阶段治疗方式主要为静脉溶栓和血管内介入治疗,2种处理方法的疗效均高度依赖于时间[1-2]。2018年美国心脏病协会/美国卒中协会(AHA/ASA)推荐急性前循环大动脉闭塞性脑梗死患者治疗时间窗为24 h内。因此,在院前阶段识别急性大血管闭塞显得格外重要。我国实施分级诊疗体系,静脉溶栓可以在初级卒中中心进行,而血管内介入治疗只能在综合卒中中心进行[1-2]。在院前分级诊疗阶段或急诊阶段对此类患者进行简单而准确的评估才能缩短从发病到实施血管内介入治疗的时间。院前急性卒中严重程度量表(PASS)由Hastrup于2016年制定,包括意识水平、凝视及肢体运动,但该量表不能准确评估大动脉闭塞性脑梗死,因而在此基础上,本研究设计了改良PASS(mPASS)以提高对急性大动脉闭塞性脑梗死患者的识别率,以便争取更多时间提高救治率,更好地服务临床。
对象与方法
一、研究对象
二、临床资料采集
采集患者临床资料:①一般情况,年龄、性别、发病时间;②既往病史,高血压、高脂血症、糖尿病、心房颤动等;③实验室数据,入院时的血清葡萄糖、LDL-C和同型半胱氨酸水平;④入院时的神经功能评分,美国国立卫生研究院卒中量表(NIHSS)、PASS及mPASS评分;⑤临床预后评估,出院3个月的改良Rankin量表(mRS)评分[5]。
三、mPASS和PASS评分细则
NIHSS评分细则于美国国立卫生研究院官方网站获取。mPASS和PASS评分细则见表1。基于病理生理学考虑,急性大动脉闭塞性脑梗死发生时,皮质症状如失语及凝视是较运动症状(如肢体瘫痪)更为特异的症状,因此本研究在PASS评分的基础上增加了提示皮质症状的指标——失语。然而在急诊评估中,非神经科医师往往不能准确区分言语含糊为失语还是构音障碍,并且部分右侧半球梗死患者的皮层损伤症状也可表现为构音障碍,因此将失语与构音障碍一同列入语言功能项目,从而得到mPASS。mPASS评分由2位从事临床工作超过10年的经验丰富的神经科医师根据患者的NIHSS评分计算得出。
表1 mPASS和PASS评分细则区分表 单位:分
| 项 目 | mPASS评分 | PASS评分 |
|---|---|---|
| 意识水平 | ||
| 清醒 | 0 | 0 |
| 嗜睡、昏睡 | 1 | 1 |
| 昏迷 | 2 | 1 |
| 凝视 | ||
| 正常 | 0 | 0 |
| 部分凝视麻痹 | 1 | 1 |
| 被动或完全凝视麻痹 | 2 | 1 |
| 肢体运动 | ||
| 正常 | 0 | 0 |
| 上肢无力 | 1 | 1 |
| 语言功能 | ||
| 正常 | 0 | - |
| 失语/构音障碍 | 1 | - |
四、统计学处理
采用SPSS 22.0进行统计学分析。正态分布计量资料采用
结果
一、闭塞组和非闭塞组急性脑梗死患者的基线资料
闭塞组与非闭塞组的基线资料具可比性(P 均> 0.05),见表2。
表2 闭塞组与非闭塞组急性脑梗死患者基线资料比较
| 项 目 | 闭塞组(59例) | 非闭塞组(53例) | t/ χ2值 | P值 |
|---|---|---|---|---|
| 年龄/岁 | 67.95±13.98 | 67.35±11.16 | 0.150 | 0.882 |
| 男性/例(%) | 32(54.2) | 31(58.5) | 0.205 | 0.651 |
| 发病时间/ h | 3.90±1.88 | 3.33±1.46 | 1.079 | 0.287 |
| 基础疾病/例(%) | 27 | 16 | ||
| 高血压 | 14(51.9) | 9(56.3) | 0.779 | 0.377 |
| 糖尿病 | 8(29.6) | 6(37.5) | 0.128 | 0.721 |
| 高脂血症 | 3(11.1) | 0(0.0) | - | 0.281 |
| 心房颤动 | 2(7.4) | 1(6.3) | - | 1.000 |
| 实验室检查 | ||||
| 血清葡萄糖/(mmol/L) | 8.35±2.64 | 7.17±2.03 | 1.679 | 0.123 |
| LDL /(mmol/L) | 2.45±0.77 | 2.66±0.79 | 0.856 | 0.397 |
| 同型半胱氨酸/(μmol/L) | 11.82±2.78 | 11.24±2.62 | 0.675 | 0.504 |
二、各量表评分的预测价值
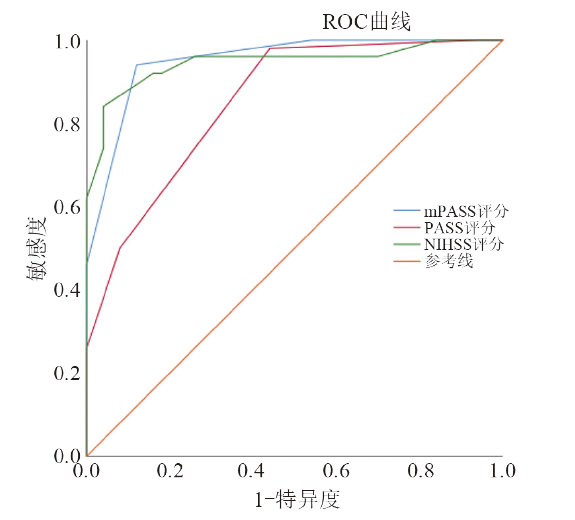
闭塞组各量表的神经功能评分均高于非闭塞组(P均< 0.01)。mPASS评分组间比较:(3.80±1.18)分 vs. (1.66±0.69)分,t = 11.091,P < 0.001;PASS评分组间比较:(2.88±1.12)分 vs. (1.46±0.73)分,t = 7.505,P < 0.001;NIHSS评分组间比较:(15.90±4.75)分 vs. (6.95±3.55)分,t = 12.692,P < 0.001;闭塞组不同评分量表的ROC曲线见图1。
图1
mPASS评分的AUC为0.951,PASS评分的AUC为0.852,NIHSS评分的AUC为0.946,mPASS vs. PASS,P = 0.014; mPASS vs. NIHSS,P = 0.089; NIHSS vs. PASS,P = 0.023。见表3 。
表3 不同量表预测价值比较
| 量 表 | 临界点 | 敏感度 | 特异度 | 约登指数 | AUC | 95%CI | 准确率 |
|---|---|---|---|---|---|---|---|
| mPASS | 2.5 | 94% | 88% | 82% | 0.951 | 0.913~0.990 | 94% |
| PASS | 1.5 | 98% | 56% | 54% | 0.852 | 0.780~0.924 | 88% |
| NIHSS | 12.5 | 84% | 96% | 80% | 0.946 | 0.899~0.993 | 84% |
以大动脉是否闭塞为因变量,mPASS评分各子项(意识水平、凝视、肢体运动、语言功能)为自变量的logistic回归结果显示凝视及语言功能均为P < 0.05。见表4。
表4 大动脉闭塞与mPASS子项的关系
| mPASS子项 | 回归系数 | 标准误 | Wald | P值 | OR值 | 95%CI |
|---|---|---|---|---|---|---|
| 凝视 | 2.520 | 0.740 | 11.603 | 0.001 | 12.429 | 2.915~52.987 |
| 语言功能 | 2.027 | 0.848 | 5.723 | 0.017 | 7.595 | 1.442~39.990 |
三、mPASS各子项与预后的关系
闭塞组的mRS评分高于非闭塞组,2组的评分为[3(5,2)]分 vs.[ 1(2,1)]分,组间比较Z = -4.729,P = < 0.001。以mRS评分为因变量,mPASS各子项为自变量进一步分析闭塞组mPASS各子项与预后的关系,结果显示F = 25.361,P < 0.001,回归方程有意义。mRS评分= 0.554+0.037意识水平+1.965凝视+0.206肢体运动+0.193语言功能,各子项与预后呈正相关。见表5。
表5 mRS评分与mPASS子项的关系
| 项 目 | 回归系数 | 标准误 | t值 | P值 |
|---|---|---|---|---|
| 常量 | 0.554 | 0.539 | 1.028 | 0.308 |
| 意识水平 | 0.037 | 0.222 | 0.169 | 0.867 |
| 凝视 | 1.965 | 0.216 | 9.105 | < 0.001 |
| 肢体运动 | 0.206 | 0.447 | 0.460 | 0.647 |
| 语言功能 | 0.193 | 0.404 | 0.478 | 0.634 |
讨论
大动脉闭塞主要指颈内动脉系统、大脑中动脉(以M1、M2段为主)、大脑前动脉(以A1段为主)和椎-基底动脉系统(以基底动脉为主)的闭塞。目前用于预测急性大动脉闭塞性脑梗死的评估工具众多,然而到目前为止尚没有足够精简、快速、敏感且被广泛接受的一种量表。NIHSS评分是现今用于评估脑卒中严重程度最常用的工具,已有多项研究表明其与大血管闭塞存在正相关关系,即NIHSS评分越高,大血管闭塞可能性越大[6-7]。但由于其评分细则冗长、操作不便等实际问题,不适用于院前120急救,且非神经科医师也存在使用困难的问题。此外,右侧大脑半球大动脉闭塞可能会出现轻至中度诸如意识障碍、肢体无力等症状,而使用NIHSS时因缺乏针对性,可能会忽略了这些症状。PASS评分内容包括皮质症状及运动症状,可用于简单评估大动脉闭塞性脑梗死,但该量表各子项分值单一且没有权重性,对更具有特异性的皮质症状没有针对性描述,不能准确地评估大动脉闭塞性脑梗死,在本研究中,与NIHSS评分和 PASS评分相比, mPASS评分的AUC显示出更高的预测值,并且特异度、约登指数、准确率均高于PASS评分,表明mPASS对急性大动脉闭塞性脑梗死的辨别能力更强,或可成为识别此类患者的有效工具。
与现有的几种量表相比,mPASS有以下优点。首先,mPASS中的各子项易于观察和有助于进行客观评估。意识水平是客观的,而失语/构音障碍是在PASS的基础上新增加的子项目[8]。Nair等[9]指出,早期脑卒中患者的脑功能受损涉及 Wernicke 区和其他语言处理相关区域,皮层下结构如尾状核参与了语言产生过程,脑卒中后这些区域之间的联系减弱,导致患者出现行为异常。Suzuki等[10]开发了一种基于皮质症状的院前评估简表来筛查适合接受血管内治疗的大血管闭塞患者,该量表中将语言功能作为预测因子之一。张运等[11]亦认为言语具有重要的预测价值,他们采用凝视、面、臂、言语、时间(G-FAST)量表对急性前循环大动脉闭塞性脑卒中患者进行早期识别,并指出在条件允许时可考虑对G-FAST高分患者尽早实施血管内治疗。本研究将语言功能作为mPASS的子项目,并证实其具有预测大动脉闭塞性脑梗死的作用,与上述研究结果一致。在紧急情况下医护人员较易观察到“凝视”,但这在其他量表中未能体现,如动脉闭塞快速评价量表(RACE)、视力-失语-忽视量表(VAN)和卒中急诊评估及分类转运(FAST-ED)量表[12-13]。其次,不同量表,包括三项内容卒中量表(3I-SS)、洛杉矶运动量表(LAMS)主要针对运动症状(肢体无力),而mPASS主要针对皮质症状(凝视、语言功能),因为运动症状也可能出现在腔隙性脑梗死中,因此上述其他量表可能不能很好地预测大动脉闭塞。本研究通过logistic回归分析证实了皮质症状之一的“凝视”是大动脉闭塞性脑梗死的危险因素,这与张运等[11]研究的结果一致, 他们认为“凝视”对预测大动脉闭塞性脑梗死具有重要价值。上述理论依据也支持本研究在PASS上增加针对皮质症状的子项。本研究还表明,mPASS的各子项与预后呈正相关,这也为后续建立急性大动脉闭塞性脑梗死预后预测模型奠定了基础。
本研究存在以下局限性:①研究为回顾性研究,且为单中心研究,后续需要收集多中心的数据加以验证。②mPASS的评分范围局限(0~6分),对患者之间的微小差异和患者临床表现的微小变化不敏感。③mPASS在不同大动脉闭塞的预测价值是否存在差异,有待进一步的研究及考证。
综上所述,本研究的mPASS或可成为一种易于记忆且有效地识别急性大动脉闭塞性脑梗死的简易工具,有助于作出更准确的分流决策并更好地实施分级诊疗。关于 mPASS 在院前急救的实用性和准确性及其预测预后的能力,仍需进一步深入研究。
参考文献
Comparative study on the efficacy and safety of alteplase and urokinase in the treatment of acute cerebral infarction
Treatment efficacy of arterial urokinase thrombolysis combined with mechanical thrombectomy for acute cerebral infarction and its influence on neuroprotective factors and factors for neurological injury
This study was designed to explore the treatment efficacy of arterial urokinase thrombolysis combined with Solitaire AB stent for acute cerebral infarction (ACI) and its influence on neuroprotective factors and factors for neurological injury.We randomly assigned 90 patients with ACI to receive arterial urokinase thrombolysis combined with Solitaire AB stent thrombectomy (observation group, OG) or to receive arterial urokinase thrombolysis (control group, CG). The two groups were compared in the National Institutes of Health Stroke Scale (NIHSS) score, activities of daily living (ADL) score, vascular recanalization rate 1 month after treatment, and serum levels of neuroprotective factors (insulin-like growth factor-I (IGF-1), neurotrophic factor (NTF), vascular endothelial growth factor (VEGF), and brain-derived neurotrophic factor (BDNF)) and factors for neurological injury (neuron-specific enolase (NSE), S100B protein (S100B), ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1), glial fibrillary acidic protein (GFAP)) before treatment and the day after treatment.The overall treatment response rate and vascular recanalization rate 1 month after treatment were markedly higher in OG than in CG (P<0.05). The NIHSS score decreased and the ADL score in both groups increased after treatment, with a lower NIHSS score and a higher ADL score in OG than in CG (all P<0.001). The difference in the complication rate between the two groups was not statistically significant (P>0.05). The day after treatment, serum levels of IGF-1, NTF, VEGF, and BDNF in both groups increased while levels of NSE, S100B, UCH-L1, and GFAP in them decreased, with higher levels of IGF-1, NTF, VEGF, and BDNF, and lower levels of NSE, S100B, UCH-L1, and GFAP in OG than in CG (all P<0.05).Arterial urokinase thrombolysis combined with Solitaire AB stent thrombectomy can enhance the treatment efficacy for ACI, stimulate the release of neuroprotective factors, and suppress the release of factors for neurological injury, without aggravating the treatment risk.AJTR Copyright © 2021.
American heart association/American stroke association deletes sections from 2018 stroke guidelines
DOI:10.5811/westjem.2018.9.39659
PMID:30429926
[本文引用: 1]

The updated American Heart Association (AHA)/American Stroke Association (ASA) Guidelines for the Early Management of Patients with Acute Ischemic Stroke were published in January 2018.1 The purpose of the guidelines is to provide an up-to-date, comprehensive set of recommendations for clinicians caring for adult patients with acute arterial ischemic stroke in a single document. The guidelines detail new and updated recommendations that reflect and incorporate the most recent literature in the evaluation and management of acute ischemic stroke. Some sections of the latest guidelines have sparked debate in the medical community. Debate with regard to deciding the optimal diagnostic and treatment strategy for patients is healthy and anticipated with the release of new medical literature or recommendations. However, what is somewhat puzzling and unanticipated with the release of these new guidelines is that within two months of their release the AHA/ASA rescinded its recently released guidelines, publishing a "correction" in which several parts of the document have been deleted.2 An action such as this at the guideline level is unprecedented in recent history and has left stakeholders in the medical community somewhat confused as to the rationale for its occurrence. This article will inform the emergency medicine (EM) healthcare professional of the recent correction of the updated stroke guidelines, identify which sections have been removed (deleted), and will provide a brief summary of the pertinent updates (that have not been deleted) to the 2018 stroke guidelines that have particular relevance to the EM community.
Validation of the simplified modified rankin scale questionnaire
DOI:10.1159/000510721
PMID:33027792
[本文引用: 1]

The modified Rankin scale (mRS) is the most common assessment tool for measuring overall functional outcome in stroke studies. The traditional way of using mRS face-to-face is time- and cost-consuming. The aim of this study was to test the validity of the Swedish translation of the simplified modified Rankin scale questionnaire (smRSq) as compared with the mRS assessed face-to-face 6 months after a stroke.Within the ongoing EFFECTS trial, smRSq was sent out to 108 consecutive stroke patients 6 months after a stroke. The majority, 90% (97/108), of the patients answered the questionnaire; for the remaining 10%, it was answered by the next of kin. The patients were assessed by face-to-face mRS by 7 certified healthcare professionals at 4 Swedish stroke centres. The primary outcome was assessed by Cohen's kappa and weighted kappa.There was good agreement between postal smRSq, answered by the patients, and the mRS face-to-face; Cohen's kappa was 0.43 (CI 95% 0.31-0.55), weighted kappa was 0.64 (CI 95% 0.55-0.73), and Spearman rank correlation was 0.82 (p < 0.0001). In 55% (59/108), there was full agreement, and of the 49 patients not showing exact agreement, 44 patients differed by 1 grade and 5 patients had a difference of 2 grades.Our results show good validity of the postal smRSq, answered by the patients, compared with the mRS carried out face-to-face at 6 months after a stroke. This result could help trialists in the future simplify study design and make multicentre trials and quality registers with a large number of patients more feasible and time-saving.© 2020 S. Karger AG, Basel.
Outcome of patients with large vessel occlusion in the anterior circulation and low NIHSS score
DOI:10.1007/s00415-020-09744-0
PMID:32062782
[本文引用: 1]

Optimal management of patients with large vessel occlusion (LVO) and low NIHSS score is unknown, which was the aim to investigate in this study.This is a retrospective analysis of a prospective single tertiary care centre 14-year cohort of patients with LVO in the anterior circulation and NIHSS score ≤ 5 on admission. Outcome was analysed according to primary intended therapy.Among 185 patients (median age 67.4 years), 52.4% received primary conservative therapy (including 26.8% secondary reperfusion in case of secondary neurological deterioration), 12.4% IV thrombolysis (IVT) only and 35.1% primary endovascular therapy (EVT). 95 (51.4%) patients experienced neurological deterioration until 3 months. Primary-IVT-only and primary-EVT compared to conservative-therapy patients had better 3 months' outcome (54.5% vs. 30.8%: OR 6.02; p = 0.004 for mRS 0-1 and 54.7% vs. 30.8%: OR 5.09; p = 0.002, respectively). Also mRS shift analysis favored primary-IVT-only and primary-EVT patients (OR 6.25; p = 0.001 and OR 3.14; p = 0.003). Outcome in primary-IVT-only vs. primary-EVT patients did not differ significantly. Patients who received secondary EVT because of neurological deterioration after primary-conservative-therapy had worse 3 months' outcome than primary-EVT patients (20.8% vs. 30.8%: OR 0.24; p = 0.047 for mRS 0-1 and OR 0.31; p = 0.019 in mRS shift analysis). Survival and symptomatic intracranial haemorrhage did not differ amongst groups.Our data indicate that primary IVT and/or EVT may be better than primary conservative therapy in patients with LVO in the anterior circulation and low NIHSS score. Furthermore, primary EVT was better than secondary EVT in case of neurological deterioration. There is an unmet need for RCTs to find the optimal therapy for this patient group.
Detailed severity assessment of Cincinnati Prehospital Stroke Scale to detect large vessel occlusion in acute ischemic stroke
DOI:10.1186/s12873-020-00360-9
PMID:32831019
[本文引用: 1]

Selecting stroke patients with large vessel occlusion (LVO) based on prehospital stroke scales could provide a faster triage and transportation to a comprehensive stroke centre resulting a favourable outcome. We aimed here to explore the detailed severity assessment of Cincinnati Prehospital Stroke Scale (CPSS) to improve its ability to detect LVO in acute ischemic stroke (AIS) patients.A cross-sectional analysis was performed in a prospectively collected registry of consecutive patients with first ever AIS admitted within 6 h after symptom onset. On admission stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) and the presence of LVO was confirmed by computed tomography angiography (CTA) as an endpoint. A detailed version of CPSS (d-CPSS) was designed based on the severity assessment of CPSS items derived from NIHSS. The ability of this scale to confirm an LVO was compared to CPSS and NIHSS respectively.Using a ROC analysis, the AUC value of d-CPSS was significantly higher compared to the AUC value of CPSS itself (0.788 vs. 0.633, p < 0.001) and very similar to the AUC of NIHSS (0.795, p = 0.510). An optimal cut-off score was found as d-CPSS≥5 to discriminate the presence of LVO (sensitivity: 69.9%, specificity: 75.2%).A detailed severity assessment of CPSS items (upper extremity weakness, facial palsy and speech disturbance) could significantly increase the ability of CPSS to discriminate the presence of LVO in AIS patients.
Functional connectivity changes in the language network during stroke recovery
DOI:10.1002/acn3.165 URL [本文引用: 1]
Emergent large vessel occlusion screen is an ideal prehospital scale to avoid missing endovascular therapy in acute stroke
DOI:10.1161/STROKEAHA.118.022107
PMID:30354974
[本文引用: 1]

Background and Purpose- The strong evidence of endovascular therapy in acute ischemic stroke patients with large vessel occlusion (LVO) is revealed. Such patients are required to direct transport to the hospital capable of endovascular therapy. There are several prehospital scales available for paramedics to predict LVO. However, they are time consuming, and several of them include factors caused by other types than LVO. Therefore, we need a fast, simple, and reliable prehospital scale for LVO. Methods- We developed a new prehospital stroke scale, emergent large vessel occlusion (ELVO) screen, for paramedics to predict LVO. The study was prospectively performed by multistroke centers. When paramedics referred to stroke center to accept suspected stroke patients, we obtain the following information over the telephone. ELVO screen was designed focusing on cortical symptoms: 1 observation; presence of eye deviation and 2 questions; paramedics show glasses, what is this? and paramedics show 4 fingers, how many fingers are there? If the presence of eye deviation or ≥1 of the 2 items were incorrect, ELVO screen was identified as positive. We evaluated between results of ELVO screen and presence of LVO on magnetic resonance angiography at hospital arrival. Results- A total of 413 patients (age, 74±13 years; men, 234 [57%]) were enrolled. Diagnosis was ischemic stroke, 271 (66%); brain hemorrhage 73 (18%); subarachnoid hemorrhage, 7 (2%); and not stroke, 62 (15%). One hundred fourteen patients had LVO (internal carotid artery, 33 [29%]; M1, 52 [46%]; M2, 21 [18%]; basilar artery, 5 [4%]; P1, 3 [3%]). Sensitively, specificity, positive predictive value, negative predictive value, and accuracy for ELVO screen to predict LVO were 85%, 72%, 54%, 93% and 76%, respectively. Among 233 patients with negative ELVO screen, only 17 (7%) had LVO, which indicated to be an ideal scale to avoid missing endovascular therapy. Conclusions- The ELVO screen is a simple, fast, and reliable prehospital scale for paramedics to identify stroke patients with LVO for whom endovascular therapy is an effective treatment.
Correlation analysis of Trial of Org 10172 in acute stroke treatment classification and National Institutes of Health Stroke Scale score in acute cerebral infarction with risk factors
DOI:10.1590/1806-9282.20210413 URL [本文引用: 1]
Stroke vision, aphasia, neglect (VAN) assessment-a novel emergent large vessel occlusion screening tool: pilot study and comparison with current clinical severity indices
DOI:10.1136/neurintsurg-2015-012131
PMID:26891627
[本文引用: 1]

Identification of emergent large vessel occlusion (ELVO) stroke has become increasingly important with the recent publications of favorable acute stroke thrombectomy trials. Multiple screening tools exist but the length of the examination and the false positive rate range from good to adequate. A screening tool was designed and tested in the emergency department using nurse responders without a scoring system.The vision, aphasia, and neglect (VAN) screening tool was designed to quickly assess functional neurovascular anatomy. While objective, there is no need to calculate or score with VAN. After training participating nurses to use it, VAN was used as an ELVO screen for all stroke patients on arrival to our emergency room before physician evaluation and CT scan.There were 62 consecutive code stroke activations during the pilot study. 19 (31%) of the patients were VAN positive and 24 (39%) had a National Institutes of Health Stroke Scale (NIHSS) score of ≥6. All 14 patients with ELVO were either VAN positive or assigned a NIHSS score ≥6. While both clinical severity thresholds had 100% sensitivity, VAN was more specific (90% vs 74% for NIHSS ≥6). Similarly, while VAN and NIHSS ≥6 had 100% negative predictive value, VAN had a 74% positive predictive value while NIHSS ≥6 had only a 58% positive predictive value.The VAN screening tool accurately identified ELVO patients and outperformed a NIHSS ≥6 severity threshold and may best allow clinical teams to expedite care and mobilize resources for ELVO patients. A larger study to both validate this screening tool and compare with others is warranted.Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.